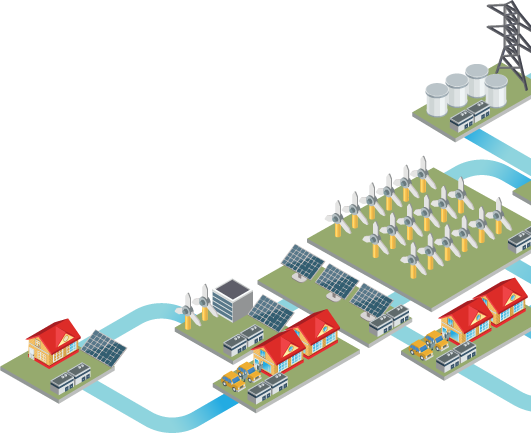
Bromine and Energy Storage
ENERGY STORAGE
A KEY ENABLER FOR LOW CARBON ENERGY SYSTEMS
 With the global population expected to grow by two billion people to reach 9.7 billion by 2050, the World Energy Council is predicting an almost twofold increase in global energy demand over the period1.
With the global population expected to grow by two billion people to reach 9.7 billion by 2050, the World Energy Council is predicting an almost twofold increase in global energy demand over the period1.
The 2015 Paris Climate Agreement saw the global community commit to avoiding global temperature increases by reducing greenhouse gas emissions. On its way to decarbonisation, and to be able to meet growing demand, the electricity sector is undergoing a major transformation as economies and consumers move away from fossil-energy-based power systems towards climate- friendly systems with an increased production and use of renewable energy.
As the supply of renewable energy grows, energy storage becomes more important. The production of electricity from wind and solar can vary significantly throughout the day. As a result, electricity is not always consumed at the time it is produced.
By storing, we can use clean electricity when we need it. Energy storage will play a key role in enabling economies globally to accelerate the energy transition. Currently there is limited storage of electricity in global electricity systems.
On average only 30 minutes of electrical energy storage is available compared to 30 days for oil2.
Demand for energy storage is therefore expected to increase as the supply of renewable energy grows. By expanding energy storage capacity, we will be able to fully exploit the growing capacities of renewable energy for a range of energy service applications from grid balancing to facility energy management.
RENEWABLE ENERGY
USE IT OR LOSE IT – UNLESS YOU STORE IT
Since the mid-2000s the EU has been proactively diversifying its energy mix with a strong focus on renewable energy. In 2015 the total share of renewable energy in final energy consumption in the EU was 15.3%, up from 8.7% in 20053.
The increase in renewable power raises new challenges to the European grid systems. The current grid capacity is strained or even insufficient to cope with the growing volumes of renewable power, as well as the increasing demand from industry and consumers for more renewable power.
Renewable energy also tends to be variable and intermittent, which can make it difficult to maintain a continuous supply to the grid. In times of low demand and high renewable electricity production wind turbines or solar panels are “switched off”.
This is what is called curtailment and represents a missed opportunity to generate more clean energy.
The case for expansion of energy storage is therefore key to efficient and sustainable management of low-carbon, renewable energy resources.
In 2014 alone some 1.5 Twh of energy produced in Germany was not used due to the incapacity of the grid system to utilize all the newly generated renewable power
This is enough energy to power 375,000 homes for a year.
ENERGY STORAGE TECHNOLOGIES
Energy storage allows electrical systems to utilize renewable energy without the need for a continuous connection to the grid. Locally, it can improve the management of distribution networks, reducing costs and improving efficiency. It can also give customers freedom to manage their own power needs.
Today there are several energy storage solutions available ranging from pumped hydro-power to battery technologies.
BROMINE-BASED ENERGY STORAGE TECHNOLOGIES
Bromine-based storage technologies are a highly efficient and cost-effective electro-chemical energy storage solution, providing a range of options to successfully manage energy from renewable sources, minimizing energy loss, reducing overall energy use and cost and safeguarding security of supply.
Typical bromine-based flow batteries include zinc-bromine (Zn-Br) and more recently hydrogen bromide (HBr).
Other variants in flow battery technology using bromine are also under development. Bromine-based storage technologies are typically used in stationary storage applications for grid,
facility or back-up/stand-by storage.
THE ADVANTAGES OF BROMINE-BASED TECHNOLOGIES
Today, there are some large sites around the world using Bromine flow batteries in order to help balancing energy deficiency. For example, the US Department of Defense uses zinc-bromine batteries for its military microgrid at the Marine Corps Air Station in Miramar California. The bromine-based technology, provided by Primus Power, was chosen for its extended duration, reliability and low cost after detailed and has helped to improve energy security, promote energy efficiency and reduce reliance on fossil fuels.
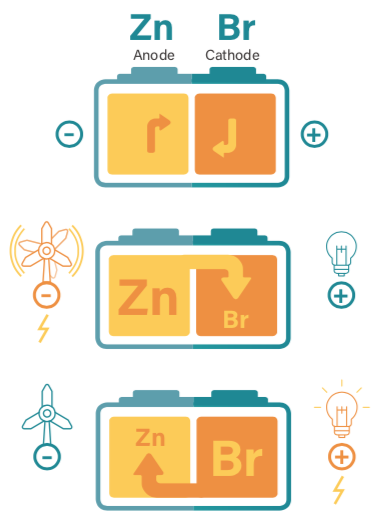
HOW DOES THE ZINC-BROMINE FLOW BATTERY WORK?
Typical bromine-based energy storage technologies are based on redox flow (after reduction–oxidation), principles. In effect, they are a rechargeable battery consisting of one or two tanks that contain chemicals dissolved in liquids and which are usually separated by a membrane.
When the two solutions flow from one tank to the other, they generate a charge by moving electrons back and forth between the tanks, thus creating energy. A flow battery is technically similar to a fuel cell and an electrochemical accumulator cell (electrochemical reversibility).
Flow batteries can be recharged rapidly by replacing the electrolyte, which is stored outside the cell. These rechargeable batteries can be left fully discharged indefinitely.
Flow batteries can be recharged rapidly by replacing the electrolyte which is stored outside the cell. These rechargeable batteries can be left fully discharged indefinitely.
The zinc–bromine flow battery is a hybrid flow battery fuelled by the reaction between zinc and bromide.
The battery is composed of two compartments. A zinc anode and a bromine cathode, divided by a porous membrane and aqueous zinc bromide flows through them.
When electricity is stored, it reacts with the zinc bromide solution, forming bromine on the battery electrodes and electroplating the zinc.
When electricity is being used, the electro-chemical reaction between zinc and bromine is reversed. Electricity is produced and the zinc bromide solution is reformed.